14th October 2023 (10 Topics)
Context:
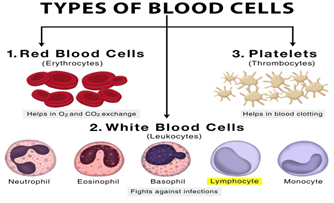
Recently, a Mumbai-based Immunoadoptive Cell Therapy Private Limited (ImmunoACT) announced the approval of India’s first chimeric antigen receptor (CAR) T-cell therapy by the Central Drugs Standard Control Organization (CDSCO) for treating leukaemias (cancers arising from the cells that produce white blood cells) and lymphomas (arising from the lymphatic system).
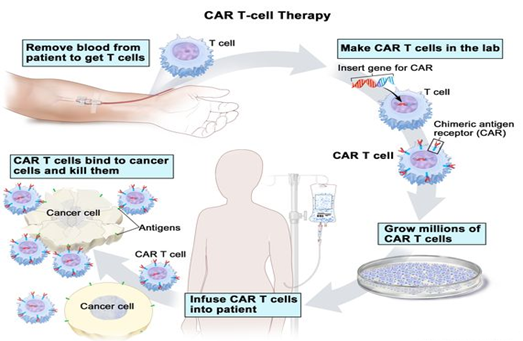
What is Chimeric Antigen Receptor (CAR) T-cell Therapy?
- It is a type of cancer immunotherapy treatment.
- Unlike chemotherapy or immunotherapy which involve taking drugs, CAR T-cell therapies use a patient's own cells.
- They are modified in the laboratory to activate T-cells and target tumor cells.
- Procedure:
- T cells are taken from a patient’s blood and then the gene for a special receptor that binds to a certain protein on the patient’s cancer cells is added to the T cells in the laboratory.
- The special receptor is called a chimeric antigen receptor (CAR). Large numbers of the CAR T cells are grown in the laboratory and given to the patient by infusion.
|
What are T Cells?
|
Need for such T-cell Therapy:
- Systemic therapy such as chemotherapy, which attacks cancer cells due to their fast growth.
- Chemotherapy drugs have limited success and significant side effects because they affect many types of cells in the body.
- Other treatments also known as immunotherapy, which work by binding to specific targets on the cancer or immune cells supporting its growth.
- This approach is less toxic as it affects fewer non-tumor cells, but only works on tumours that have these targets.
- Thus, using own living cell becomes an idea.
|
Role of Central Drugs Standard Control Organization (CDSCO)
|