

26th June 2025 (28 Topics)
Context
In a medical first in India, a newborn in Kerala’s SAT Hospital, Thiruvananthapuram, is being treated before symptoms appear for Spinal Muscular Atrophy (SMA) — a rare genetic neuromuscular disorder. The treatment was made possible due to prenatal genetic screening which identified that the infant carries the SMN1 gene mutation linked to SMA.
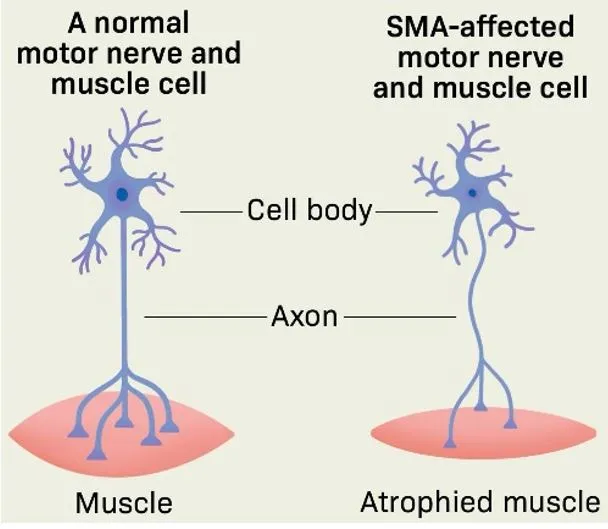
What is Spinal Muscular Atrophy (SMA)?
- Spinal Muscular Atrophy (SMA) is a hereditary disorder affecting motor neurons — the nerve cells that control muscle movement.
- It leads to progressive muscle weakness and can be life-threatening, especially in infants.
- It is caused by mutations in the SMN1 gene, usually inherited from both parents.
- It is currently incurable, but early treatment can delay or reduce symptom severity.
- What makes this case special? The mother, an SMA patient, underwent prenatal screening, which detected the SMN1 mutation in the foetus. Choosing to continue the pregnancy, she enabled doctors to begin Risdiplam treatment within three weeks of birth, before any symptoms appeared—a rare, early intervention in India.
About Risdiplam
|


