

- Around the world, there's a patchwork of laws addressing the possibility of editing the genomes of the human embryo.
- In Russia, germline gene modification for reproduction is not considered by the legislative legislation.
- In Canada and many European countries, bans are quite strict.
- In China, India, Japan, and Ireland bans existed but didn't necessarily have legal enforcement mechanisms behind them.
- Indian protocols prohibit human germline editing and reproductive cloning, as detailed in the National Guidelines for Stem Cell Research by the Indian Council of Medical Research.

Regulation of genome editing techniques in India:
- In India, genetically modified organisms and products thereof are regulated under the "Rules for the manufacture, use, import, export and storage of hazardous microorganisms, genetically engineered organisms or cells, 1989" (referred to as Rules, 1989) notified under the Environment (Protection) Act, 1986.
- These Rules are implemented by the Ministry of Environment, Forest and Climate Change, Department of Biotechnology and State Governments through six competent authorities.
- The Rules, 1989 are supported by a series of guidelines on contained research, biologics, confined field trials, food safety assessment, environmental risk assessment etc.
- The definition of genetic engineering in the Rules, 1989 implies that new genome engineering technologies including gene-editing technologies like CRISPR/Cas9 and gene drives may be covered under the rules.
-
- National Guidelines for Gene Therapy Product Development and Clinical Trials:
- In a recent development, the Indian Council of Medical Research (ICMR) has framed national guidelines and regulations regarding the procedures and requirements to be followed for performing Gene Therapy in India.
- Since this nascent field is emerging in India, the Government has proactively have come up with the National Guidelines for Gene Therapy Product Development and Clinical Trials to promote further research and streamline regulatory processes for future clinical trials using gene therapeutic products (GTPs).
- As per the New Drugs and Clinical trial Rules (2019) the GTPs falls under ‘new drug’ and shall always be deemed to be ‘new drug’.
- Thus as per these rules framed jointly by Indian Council of Medical Research and Department of Biotechnology (DBT), ‘academic trials’ are not applicable to clinical trials using GTPs.
-
- Gene Therapy Advisory and Evaluation Committee (GTAEC):
- The Government has also proposed to establish the Gene Therapy Advisory and Evaluation Committee (GTAEC) anchored at ICMR.
- GTAEC shall be an independent body of experts representing diverse areas of biomedical research, concerned government agencies and other stakeholders.
- This committee will be composed of a core group of scientists and clinicians in the sector, as well as representation of the government agencies (ICMR, DGHS, CDSCO, DBT, DST, MCI).
- For each disease area in GTP trials, specific clinical consultants with extensive disease-specific expertise will be co-opted to aid in the decision-making process.
- Genome editing or gene editing is a group of technologies that give scientists the ability to change an organism's DNA.
- These technologies allow genetic material to be added, removed, or altered at particular locations in the genome.

- Several approaches to genome editing have been developed. A recent one is known as CRISPR-Cas9, which is short for ‘clustered regularly interspaced short palindromic repeats’ and ‘CRISPR-associated protein 9’.
- It refers to a kind of molecular construct bacteria use to defend themselves from viral invaders.
- The CRISPR-Cas9 system has generated a lot of excitement in the scientific community because it is faster, cheaper, more accurate, and more efficient than other existing genome editing methods.
- CRISPR-Cas9 was adapted from a naturally occurring genome editing system in bacteria.
- The bacteria capture snippets of DNA from invading viruses and use them to create DNA segments known as CRISPR arrays.
- The CRISPR arrays allow the bacteria to "remember" the viruses (or closely related ones).
- If the viruses attack again, the bacteria produce RNA segments from the CRISPR arrays to target the viruses' DNA.
- The bacteria then use Cas9 or a similar enzyme to cut the DNA apart, which disables the virus.
- The CRISPR-Cas9 system works similarly in the lab. Researchers create a small piece of RNA with a short "guide" sequence that attaches (binds) to a specific target sequence of DNA in a genome.
- The RNA also binds to the Cas9 enzyme. As in bacteria, the modified RNA is used to recognize the DNA sequence, and the Cas9 enzyme cuts the DNA at the targeted location.
- Although Cas9 is the enzyme that is used most often, other enzymes (for example Cpf1) can also be used.
- Once the DNA is cut, researchers use the cell's own DNA repair machinery to add or delete pieces of genetic material, or to make changes to the DNA by replacing an existing segment with a customized DNA sequence.
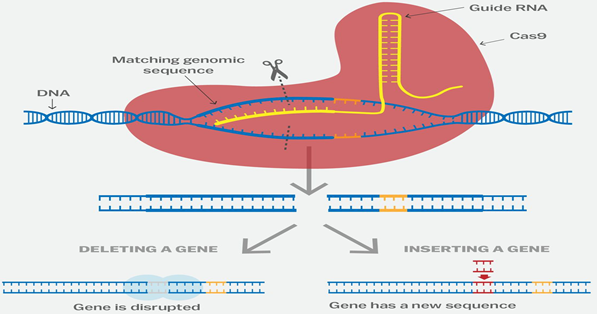
Other approaches to genome editing:
Apart from CRISPR, there are several approaches to genome editing such as Zinc finger nuclease-based engineering, TALEN, and CRISPRs, Mega nuclease-based engineering.
- Meganucleases: Meganucleases, discovered in the late 1980s, are enzymes in the endonuclease family, which are characterized by their capacity to recognize and cut large DNA sequences (from 12 to 40 base pairs).
- As opposed to meganucleases, the concept behind ZFNs and TALEN technology is based on a non-specific DNA cutting enzyme, which can then be linked to specific DNA sequence recognizing peptides such as zinc fingers and transcription activator-like effectors (TALEs).
- Zinc-finger nucleases (ZFNs) are artificial restriction enzymes generated by fusing a zinc finger DNA-binding domain to a DNA-cleavage domain.
- Transcription activator-like effector nucleases (TALENs) are artificial restriction enzymes generated by fusing a specific DNA-binding domain to a non-specific DNA cleaving domain.
What is DNA?
- Deoxyribonucleic acid (DNA) is the hereditary material in humans and almost all other organisms. Nearly every cell in a person’s body has the same DNA.
- Most DNA is located in the cell nucleus (where it is called nuclear DNA), but a small amount of DNA can also be found in the mitochondria (where it is called mitochondrial DNAor mtDNA).
- Mitochondriaare structures within cells that convert the energy from food into a form that cells can use.
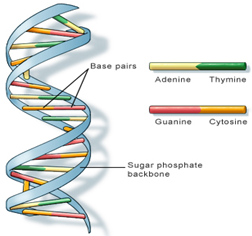
- The information in DNA is stored as a code made up of four chemical bases:
- adenine (A)
- guanine (G)
- cytosine (C)
- thymine (T)
- DNA bases pair up with each other, A with T and C with G, to form units called base pairs. Each base is also attached to a sugar molecule and a phosphate molecule.
- Together, a base, sugar, and phosphate are called a Nucleotides are arranged in two long strands that form a spiral called a double helix.
- The structure of the double helix is somewhat like a ladder, with the base pairs forming the ladder’s rungs and the sugar and phosphate molecules forming the vertical sidepieces of the ladder.
- An important property of DNA is that it can replicate, or make copies of itself.
- Each strand of DNA in the double helix can serve as a pattern for duplicating the sequence of bases.
- This is critical when cells divide because each new cell needs to have an exact copy of the DNA present in the old cell.
- Embryonic stem cell and transgenic animals: CRISPR-Cas systems can be used to rapidly and efficiently engineer one or multiple genetic changes to murine embryonic stem cells for the generation of genetically modified mice.
- Disease modelling: Disease animal models have been essential resources in advancing the biomedicine field. With the help of genome editing technologies, many applicable models with specific mutations which could mimic clinical phenotypes have been generated.
- Cancer models: With the help of genome editing tools, numerous studies have been carried out through modifying key genes for generating accurate and specific cancer models. Cancer models are the most effective ways to study mutational functions which result in cancer.
- Genome editing technologies are not only used for generating disease animal models but also destined to enter the therapeutic area. There are plentiful means for genome editing based therapy:
- inactivation or correction of harmful mutations
- introduction of protective mutations
- insertion of therapeutic exogenous genes
- destruction of viral DNA
- Productivity improvement: Continuous decrease in the availability of land and water for agriculture, uncertain weather conditions and a growing population are signals for the urgent need for an alternative approach in the country. In this scenario, scientists are optimistic about the possibilities of genome editing for enhancing crop productivity to overcome the shortcomings of traditional transgenic methods like irregular breeding cycles, lack of precision in intended trait selection and uncertainty in getting desirable mutations.
- Allergy-free food: Food allergies affect a huge percentage of the population and can be life-threatening in some cases. With CRISPR, it could be possible to make milk, eggs or peanuts that are safe for everyone to eat.
- Greener fuels: Gene editing could improve the production of biofuels by algae. Using CRISPR-Cas9, the company Synthetic Genomics has created strains of algae that produce twice as much fat, which is then used to produce biodiesel. In particular, the gene-editing tool allowed scientists to find and remove genes that limit the production of fats.
- Eradicating pests: CRISPR could help us control the numbers of animal species that transmit infectious diseases or that are invasive in a particular ecosystem. The gene-editing technology can be used to create ‘gene drives’ that ensure a genetic modification will be inherited by all the offspring, spreading throughout an animal population over several generations.
Genome editing is of great interest in the prevention and treatment of human diseases. Currently, most research on genome editing is done to understand diseases using cells and animal models. Scientists are still working to determine whether this approach is safe and effective for use in people. Its significance can be known from the following points:
- Identification: It is being explored in research on a wide variety of diseases, including single-gene disorders such as cystic fibrosis, haemophilia, and sickle cell disease.
- Treating complex diseases: It also holds promise for the treatment and prevention of more complex diseases, such as cancer, heart disease, mental illness, and human immunodeficiency virus (HIV) infection.
- Treating disorder: India has a large burden of genetic disorders and unmet medical needs and gene therapy can prove to be a turning point in the treatment of such disorders.
- Crops and Livestock: The technique of genome editing can also be used for increasing yield, introducing resistance to disease and pests, tolerance of different environmental conditions.
- Industrial Biotechnology: It can be used for developing ‘third generation’ biofuels and producing chemicals, materials, and pharmaceuticals.
- Biomedicine: Genome editing is also beneficial for pharmaceutical development, xenotransplantation, gene and cell-based therapies, control of insect-borne diseases.
- Reproduction: It helps in preventing the inheritance of a disease trait.
- Current scientific advancements show that CRISPR is not only an extremely versatile technology, but it’s also proving to be precise and increasingly safe to use. But a lot of progress still has to be made.
- At presents, scientists are only beginning to see the full potential of genome-editing tools like CRISPR-Cas9.
- Currently, there are no internationally agreed-upon laws or regulations on gene editing, leaving scientific research and application of CRISPR technology to the discretion of individual countries. An international protocol is the need of the hour.
- For CRISPR-Cas9 genome editing technology to be embraced by the public, it must be applied responsibly. The global research effort must remain focused on treating disease rather than engineering new human traits or creating so-called designer babies.
- According to a recent report, the global gene therapy market is anticipated to reach USD 4,300 million by 2021.
- The demand for gene therapy is primarily driven by continuous technological advancements and successful progression of several clinical trials targeting treatments with strong unmet need.
- Moreover, rising R&D spend on platform technologies by large and emerging biopharmaceutical companies and favourable regulatory environment will accelerate the clinical development and the commercial approval of gene therapies in the foreseeable future.
- Despite the promise, the high cost of gene therapy represents a significant challenge for commercial adoption in the forecast period.
- North America holds a dominating position in the global gene therapy market which is followed by Europe and the Asia Pacific.
- Asia Pacific region shows signs of high growth owing to the booming economies of India, and China.
- Overall, the field of gene therapy continues to mature and advance with many products in development and nearing commercialization.
Q1. What is genome editing? Discuss the current state of the science of human gene editing, as well as possible future directions and challenges.
Q2. What are the potential clinical applications that may hold promise for the treatment of human diseases?
Q3. Do current ethical and legal standards for human subject’s research adequately address human gene editing, including germ line editing? What are the ethical, legal, and social implications of the use of current and projected gene-editing technologies in humans?
Related Articles



