Context
The first gene editing technology securing approval for the treatment of sickle cell anemia and beta-thalassemia utilise the Nobel-winning CRISPR/Cas 9 genome. However, the success of this technology hinges on a sufficient commitment to integrating ethics into its application.
The approved gene therapies
- Casgevy and Lyfgenia, the two cell-based gene therapies approved by the Food and Drug Administration (FDA)for sickle cell anemia treatment and beta-thalassemia.
- They utilise the Nobel-winning CRISPR/Cas 9 genome editing technology.
- Casgevy, a cell-based gene therapy, is approved for the treatment of sickle cell disease in patients 12 years of age and older with recurrent vaso-occlusive crises. Casgevy is the first FDA-approved therapy utilizing CRISPR/Cas9, a type of genome editing technology.
- Lyfgenia is a cell-based gene therapy. Lyfgenia uses a lentiviral vector (gene delivery vehicle) for genetic modification and is approved for the treatment of patients 12 years of age and older with sickle cell disease and a history of vaso-occlusive events.
|
What is Sickle cell Anemia?
|
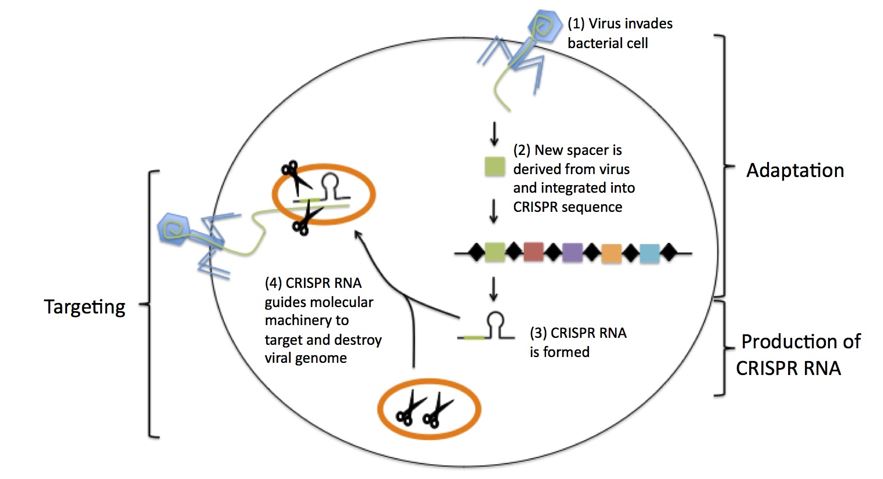
What is CRISPR?
- CRISPR stands for Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR), a hallmark of a bacterial defense system.
- Simply put, CRISPR is a technology that can be used to edit genes
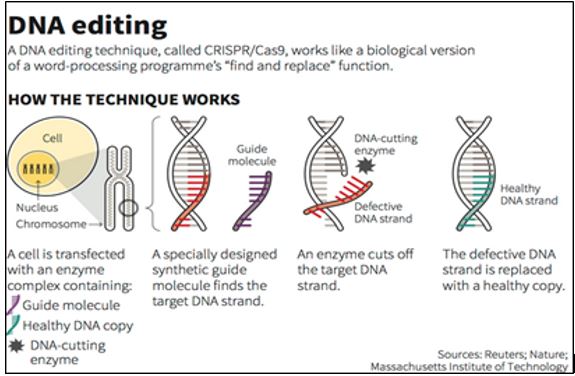
How CRISPR works?
The CRISPR-Cas9 system consists of two key molecules that introduce a change (mutation) into the DNA. These are:
- An enzyme called Cas9. This acts as a pair of ‘molecular scissors’ that can cut the two strands of DNA at a specific location in the genome so that bits of DNA can then be added or removed.
- A piece of RNA called guide RNA (gRNA). This consists of a small piece of pre-designed RNA sequence (about 20 bases long) located within a longer RNA scaffold. The scaffold part binds to DNA and the pre-designed sequence ‘guides’ Cas9 to the right part of the genome. This makes sure that the Cas9 enzyme cuts at the right point in the genome.
- The guide RNA is designed to find and bind to a specific sequence in the DNA. The guide RNA has RNA bases that are complementary to those of the target DNA sequence in the genome. This means that the guide RNA will only bind to the target sequence and no other regions of the genome.
- The Cas9 follows the guide RNA to the same location in the DNA sequence and makes a cut across both strands of the DNA.
- At this stage the cell recognises that the DNA is damaged and tries to repair it.
- Scientists can use the DNA repair machinery to introduce changes to one or more genes in the genome of a cell of interest.
What are the issues?
- Access and Affordability: CRISPR-based therapies are expensive to develop and administer.
- Inequities: Disparities in healthcare infrastructure and resources can lead to unequal distribution. Developing countries may struggle to afford or implement CRISPR therapies, perpetuating existing health inequalities.
- Global Disparities: While CRISPR research and clinical trials occur worldwide, the benefits may not reach all populations equally. High-income countries often lead in scientific advancements, leaving low-income nations lagging behind.
What are the ethical concerns in CRISPR applications?
- Off-Target Effects: CRISPR-Cas9 can inadvertently edit unintended regions of the genome. These off-target effects pose risks, especially when applied to human embryos or germline cells.
- Ensuring precision and minimizing off-target effects is critical to prevent unintended consequences.
- Germline Editing: Editing the genes of embryos or germline cells affects future generations. Ethical debates surround the permanence and unforeseen consequences of germline modifications.
- Balancing the potential benefits with long-term risks requires thoughtful consideration.
- Designer Babies: The ability to select specific traits (e.g., intelligence, appearance) raises ethical questions. Should we use CRISPR to create “designer babies” with predetermined genetic features?
- Striking a balance between medical necessity and ethical boundaries is essential.
- Informed Consent: Patients and research participants must fully understand the risks, benefits, and uncertainties of CRISPR therapies.
- Informed consent becomes complex when applied to germline editing or vulnerable populations.
- Equity in Clinical Trials: Ensuring diverse representation in clinical trials is crucial. Historically, marginalized communities have been underrepresented.
- Addressing biases and promoting inclusivity in research is essential for equitable outcomes.
- Dual-Use Dilemma: CRISPR technology has dual-use potential: it can be used for both therapeutic and harmful purposes (e.g., bioterrorism).
Regulatory Framework in India
- CRISPR research in India is governed by existing legal and regulatory frameworks.
- Gene Therapy Products (GTPs), including those developed through CRISPR, undergo thorough approval processes by regulatory bodies like the CDSCO.
- Ethical guidelines and oversight by committees such as RCGM and GEAC ensure adherence to ethical standards in biomedical research.