Environment & Ecology: Nutrient Cycles & Revision Test - I
Panchayati Raj Institutions

Safe City Project

Khasi Tribes

Khasi Accord
National Crisis Management Committee (NCMC)
-min.jpg)
Nutrient Cycles
Concept of Bio-Geochemical Cycles
- The cyclical path of elements from abiotic system to the biotic system and back is called Biogeochemical cycle.
- The chemical elements, including all the essential elements of life, tend to circulate in the biosphere in characteristic pathways from environment to organisms and back to the environment.
- These more or less circular pathways are known as biogeochemical cycles. In other words, a biogeochemical cycle is a circuit or pathway by which a chemical element or molecule moves through both biotic (“bio-”) and abiotic (“geo-”) compartments of an ecosystem. In effect, the element is recycled, although in some such cycles there may be places (called sinks) where the element is accumulated or held for a long period of time.
- The movement of these elements and inorganic compounds that are essential to life can be conveniently designated as nutrient cycling.
- The dissipation of energy in some form is always necessary to drive material cycles.
- This cycle contains any of the natural pathways by which essential elements of living matter are circulated.
- Biogeochemical cycles are named for the cycling of biological, geological and chemical elements through Earth and its atmosphere.
- The cycles move substances through the biosphere, lithosphere, atmosphere and hydrosphere. Cycles are gaseous and sedimentary.
- Gaseous cycles includes nitrogen, oxygen, carbon and water
- Sedimentary cycles includes phosphorus and sulphur
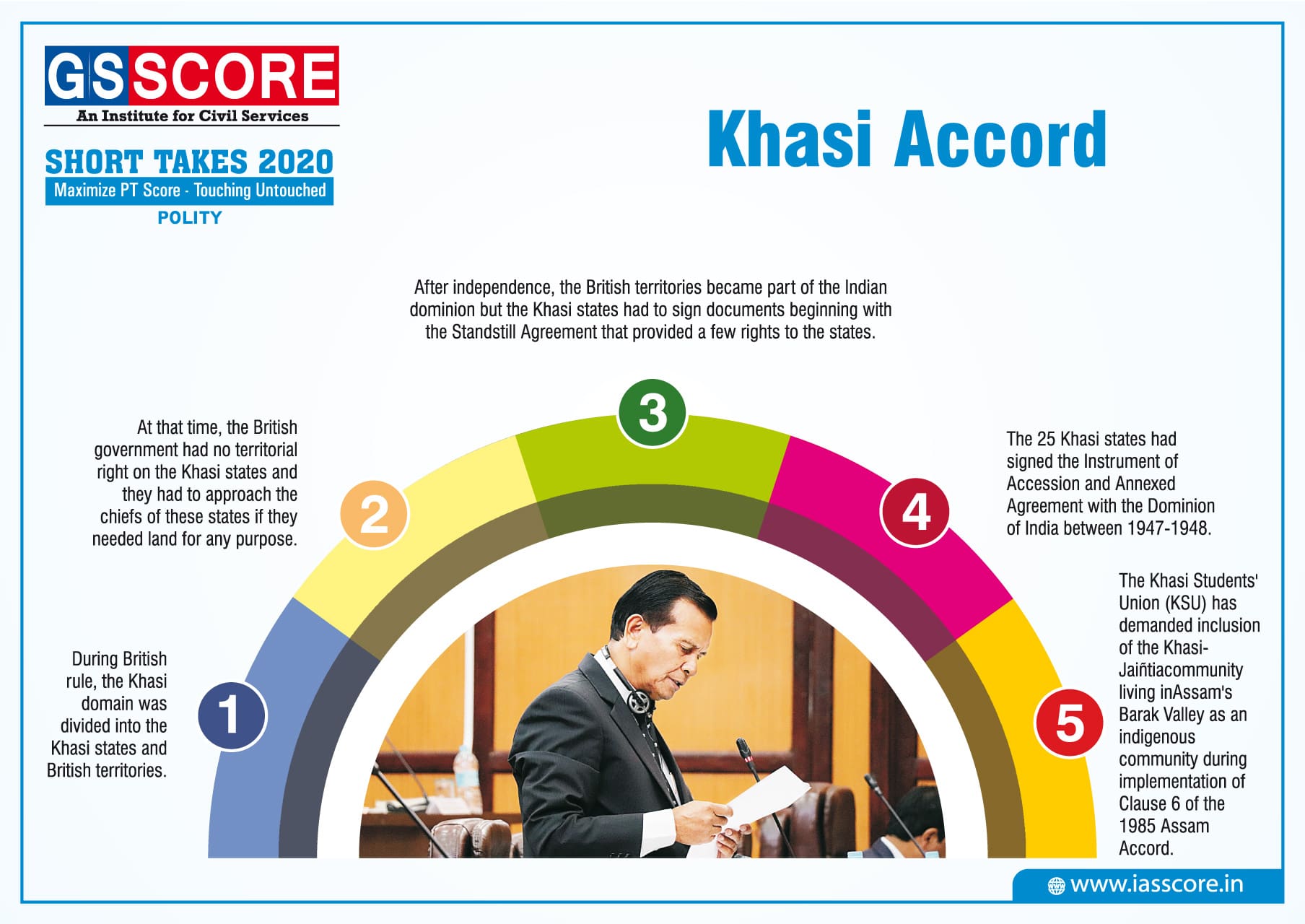
CARBON CYCLE
- Carbon is a constituent of all organic compounds, many of which are essential to life on Earth. Carbon dioxide is an atmospheric constituent that plays several vital roles in the environment.
- It is a greenhouse gas that traps infrared radiation heat in the atmosphere.
- It plays a crucial role in the weathering of rocks. It is the carbon source for plants.
- It is stored in biomass, organic matter in sediments, and in carbonate rocks like limestone.
Steps in Carbon Cycle
- Carbon enters the atmosphere as carbon dioxide from respiration and combustion.
- Carbon dioxide is absorbed by producers to make carbohydrates in photosynthesis.
- Animals feed on the plant passing the carbon compounds along the food chain. Most of the carbon they consume is exhaled as carbon dioxide formed during respiration. The animals and plants eventually die.
- The dead organisms are eaten by decomposers and the carbon in their bodies is returned to the atmosphere as carbon dioxide. In some conditions decomposition is blocked. The plant and animal material may then be available as fossil fuel in the future for combustion.
The cycle has four major reservoirs of carbon interconnected by pathways of exchange. The reservoirs are:
- The atmosphere
- The terrestrial biosphere (which usually includes freshwater systems and non-living organic material, such as soil carbon)
- The oceans (which includes dissolved inorganic carbon and living and non-living marine biota).
- The sediments (which includes fossil fuels).
The annual movements of carbon, the carbon exchanges between reservoirs, occur because of various chemical, physical, geological, and biological processes.
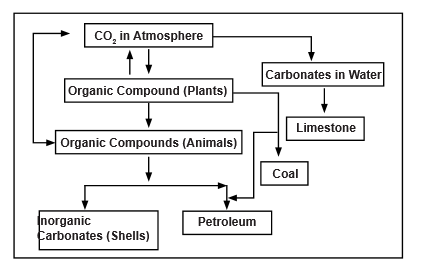
HYDROLOGICAL CYCLE
Water cycle, also called hydrologic cycle, cycle that involves the continuous circulation of water in the Earth atmosphere system. Of the many processes involved in the water cycle, the most important are evaporation, transpiration, condensation, precipitation, and runoff. Although the total amount of water within the cycle remains essentially constant, its distribution among the various processes is continually changing.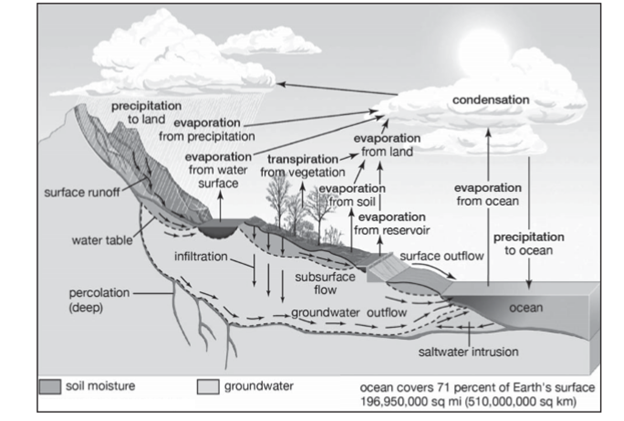
Different Steps of the Hydrological Cycle
Evaporation
- Evaporation, one of the major processes in the cycle, is the transfer of water from the surface of the Earth to the atmosphere. By evaporation, water in the liquid state is transferred to the gaseous, or vapour, state.
- This transfer occurs when some molecules in water mass have attained sufficient kinetic energy to eject themselves from the water surface. The main factors affecting evaporation are temperature, humidity, wind speed, and solar radiation.
Transpiration
- When water vapour is also discharged from plant leaves by a process called transpiration.
Sublimation
- Evaporation from snow and ice, the direct conversion from solid to vapor is known as sublimation.
Condensation
- When water vapour rises, it cools slightly and condenses. Generally, the water condenses on dust particles in the air and becomes liquid. Sometimes the water skips the liquid phase and turns directly into a solid - in the form of ice, hail, or snow. In the liquid form the particles collect and form clouds.
- Condensation may take place as soon as the air contains more water vapour than it can receive from a free water surface through evaporation at the prevailing temperature. This condition occurs as the consequence of either cooling or the mixing of air masses of different temperatures. By condensation, water vapour in the atmosphere is released to form precipitation.
Precipitation
- The condensed water vapor falling to the surface of the Earth is known as precipitation. It occurs in the form of snow, hail and rain.
Infiltration and Percolation
- When precipitation falls on the ground, some of it moves downwards into cracks, joints, and pores in the soil. The entry of water into the subsurface is termed infiltration.
- The process of percolation refers to the subsequent movement of water through subsurface soil pores until it reaches the water table. At this point it becomes groundwater. This is a slow process, which is why more water flows back to the ocean through surface runoff than groundwater discharge
Groundwater Flow
- Groundwater is water that is held in cracks and pore spaces below ground. This water can be tapped by water supply wells or continue moving below the ground until it eventually returns to the surface.
- The process by which groundwater exits the ground is known as groundwater discharge. This groundwater can either discharge directly into oceans, or more commonly, it discharges to surface water (lakes and rivers) and then travels to the ocean as surface runoff.
NITROGEN CYCLE
Nitrogen is an essential component of protein and required by all living organisms including human beings. Nitrogen is needed for our DNA, RNA and proteins and is critical to human agriculture. Nitrogen, a component of proteins and nucleic acids, is essential to life on Earth. Although 78% by volume of the atmosphere is nitrogen gas, this abundant reservoir exists in a form unusable by most organisms. Through a series of microbial transformations, however, nitrogen is made available to plants, which in turn ultimately sustain all animal life.
The steps, which are not altogether sequential, fall into the following classifications:
Nitrogen Fixation
- Nitrogen enters the living world by way of bacteria and other single-celled prokaryotes, which convert atmospheric nitrogen N2—into biologically usable forms in a process called nitrogen fixation. Some species of nitrogen-fixing bacteria are free-living in soil or water, while others are beneficial symbionts that live inside of plants.
- Nitrogen-fixing microorganisms capture atmospheric nitrogen by converting it to ammonia (NH3) which can be taken up by plants and used to make organic molecules. The nitrogen-containing molecules are passed to animals when the plants are eaten. They may be incorporated into the animal’s body or broken down and excreted as waste, such as the urea found in urine.
- Nitrogen fixation, in which nitrogen gas is converted into inorganic nitrogen compounds, is mostly (90 percent) accomplished by certain bacteria and blue-green algae (see nitrogen fixation). A much smaller amount of free nitrogen is fixed by abiotic means (e.g., lightning, ultraviolet radiation, electrical equipment) and by conversion to ammonia through the Haber-Bosch process.
- Nitrates and ammonia resulting from nitrogen fixation are assimilated into the specific tissue compounds of algae and higher plants. Animals then ingest these algae and plants, converting them into their own body compounds
Ammonification
- When plants or animal die organic nitrogen is again released back into the soil. Bacteria or fungi present in the soil convert them back into ammonium. This process is also called as
- The remains of all living things and their waste products are decomposed by microorganisms in the process of ammonification, which yields ammonia. (Under anaerobic, or oxygen-free, conditions foul-smelling putrefactive products may appear, but they too are converted to ammonia in time.) Ammonia can leave the soil or be converted into other nitrogen compounds, depending in part on soil conditions.
Nitrification
- In this process, the ammonia is converted into nitrate by the presence of bacteria in the soil. Ammonia is oxidized to form nitrites by bacteria such as Nitrosomonas species. Nitrates are converted into nitrates by Nitrobacter. This conversion is very important as ammonia gas is toxic for plants.
Denitrification
- Denitrification is the process that converts nitrate to nitrogen gas, thus removing bioavailable nitrogen and returning it to the atmosphere. Dinitrogen gas (N2) is the ultimate end product of denitrification, but other intermediate gaseous forms of nitrogen exists. Some of these gases, such as nitrous oxide (N2O), are considered greenhouse gases, reacting with ozone and contributing to air pollution.
- Unlike nitrification, denitrification is an anaerobic process, occurring mostly in soils and sediments and anoxic zones in lakes and oceans.
OXYGEN CYCLE
- Oxygen in the atmosphere is about 21%, and it is the second most abundant gas after nitrogen.
- It is mostly utilized by living organisms, especially man and animals in respiration.
- Oxygen is also the most common element of human body. Oxygen is also used during combustion, decomposition, and oxidation.
- The circulation of oxygen is through three main flow systems including the (air) atmosphere, the biosphere, and the earth’s crust.
- In the oxygen cycle, the main driving factor is photosynthesis which is the process whereby green plants and algae make their own food by use of solar energy, water, and carbon dioxide to gives off oxygen as a by-product.
- Hence, for oxygen to remain in the atmosphere, it has to circulate through various forms of nature which is essentially termed as the oxygen cycle. The circulation depends on the various activities on Earth.
Oxygen is produced by:
- Plants – Plants produce oxygen via photosynthesis
- Sunlight – Some oxygen is produced when sunlight reacts with water vapour in the atmosphere.
Oxygen is used up in:
- Respiration – All organisms use oxygen for respiration.
- Decomposing– When plants and animals die, they decompose. This process uses up oxygen and releases carbon dioxide into the air.
- Rusting – Also called oxidation, this process causes metals to rust. Also a process which uses up oxygen.
- Combustion– The process by which fire is generated also requires oxygen, along with heat and fuel. This process also uses up oxygen and releases carbon di oxide into the atmosphere.
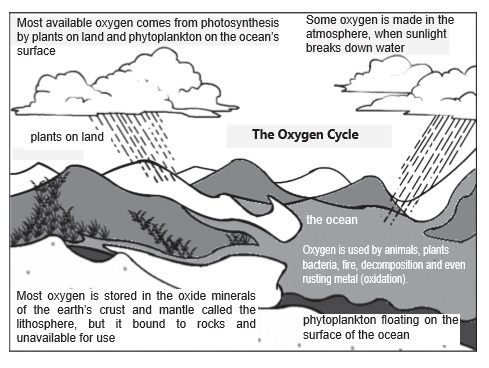
SULPHUR CYCLE
- The sulphur reservoir is in the soil and sediments where it is locked in organic (coal, oil and peat) and inorganic deposits (pyrite rock and sulphur rock) in the form of sulphates, sulphides and organic sulphur.
- It is released by weathering of rocks, erosional runoff and decomposition of organic matter and is carried to terrestrial and aquatic ecosystems in salt solution.
- The sulphur cycle is mostly sedimentary except two of its compounds, hydrogen sulphide (H2S) and sulphur dioxide (SO2), which add a gaseous component.
- Sulphur enters the atmosphere from several sources like volcanic eruptions, combustion of fossil fuels (coal, diesel etc.), from the surface of the ocean and gases released by decomposition.
- Atmospheric hydrogen sulphide also gets oxidised into sulphur dioxide.
- Atmospheric sulphur dioxide is carried back to the earth after being dissolved in rainwater as weak sulphuric acid (acid rain).
- Whatever the source, sulphur in the form of sulphates is taken up by plants and incorporated through a series of metabolic processes into sulphur bearing amino acid which is incorporated in the proteins of autotroph tissues. It then passes through the grazing food chain.
- Sulphur bound in a living organism is carried back to the soil, to the bottom of ponds and lakes and seas through excretion and decomposition of dead organic material.
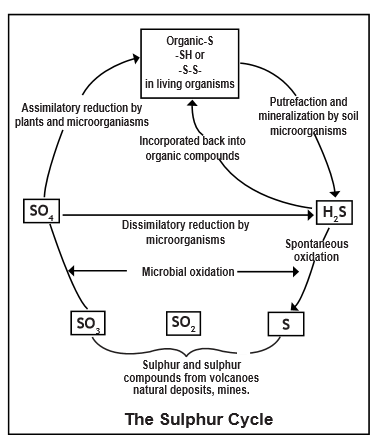
PHOSPHORUS CYCLE
- Phosphorus is an essential nutrient for plants and animals.
- It is a part of DNA molecules, of molecules that store energy (ATP and ADP) and of fats of cell membranes. Phosphorus is also a building block of certain parts of the human and animal body, such as the bones and teeth.
- Phosphorus can be found on earth in water, soil and sediments. Unlike the compounds of other matter cycles phosphorus cannot be found in air in the gaseous state. This is because phosphorus is usually liquid at normal temperatures and pressures. It is mainly cycling through water, soil and sediments. In the atmosphere phosphorus can mainly be found as very small dust particles.
- Phosphorus moves slowly from deposits on land and in sediments, to living organisms, and then much more slowly back into the soil and water sediment. The phosphorus cycle is the slowest one of the matter cycles. The phosphorus cycle appears somewhat simpler than the nitrogen cycle, because phosphorus occurs in fewer chemical forms.
Parts of the Cycle
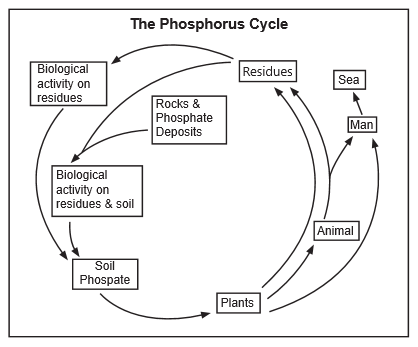
- As shown in the Figure, phosphorus, a necessary constituent of protoplasm, tends to circulate with organic compounds in the form of phosphates (PO4), which are again available to plants.
- The great reservoir of phosphorus is not the air, however, but in apatite mineral deposits formed in past geological ages (that is, in the lithosphere). Atmospheric dust and aerosols return a large amount of phosphorus (not phosphate) to the land yearly, but phosphate continually returns to the sea, where part of it is deposited in the shallow sediments and part of it is lost to the deep sediments.
- Contrary to popular belief, seabirds play only a limited role in returning phosphorus to the cycle (as shown by the guano deposits located on the coast of Peru). This transfer of phosphorus and other materials by birds from the sea to the land is continuing, likely at the same rate at which it occurred in the past - but these guano deposits have been mined out.
E-Waste Clinic in Madhya Pradesh
Context
The Bhopal Municipal Corporation (BMC) and the Central Pollution Control Board (CPCB) have signed a MOU to set up the country’s first e-waste clinic in Bhopal.
About
- Electronic waste will be collected door-to-door or could be deposited directly at the clinic in exchange for a fee.
- Door-to-door collection will happen in two ways. Either separate carts for the collection of e-waste will be designed, or separate bins will be attached to existing ones meant for solid and wet waste.
- The CPCB will provide technical support at the unit and the collected hazardous waste will then be sent to Bengaluru for recycling.
- The clinic is being conceived in compliance with the Solid Waste Management Rules, 2016.
- It would be a 3-month pilot project, which, if successful, will be replicated everywhere in India.
E- waste:
- Electronic wasteor e-waste describes discarded electrical or electronic devices. It is a term for electronic products that have become unwanted, obsolete, and have reached the end of their useful life.
- Electronic waste products have exhausted their utility value through redundancy, replacement, or breakage and include both “white goods” such as refrigerators, washing machines, and microwaves and “brown goods” such as televisions, radios, computers, and cell phones.
E-Waste (Management) Rules, 2016:
- The rules extend to Producer, consumer, collection centre, dismantler and recycler manufacturer, dealer, refurbisher and Producer Responsibility Organization (PRO). However, micro and small industries are exempted.
- The applicability of the rules extends to components, consumables, spares and parts of EEE. Further, Compact Fluorescent Lamp (CFL) and other mercury containing lamp brought are under the purview of rules.
- Collection mechanism based approach has been adopted to include collection centre, collection point, take back system etc for collection of e - waste by Producers under Extended Producer Responsibility (EPR).
Impact on Human health-
- The complex compositionand improper handling of e-waste adversely affect human health. Researchers have linked e-waste to adverse effects on human health, such as inflammation and oxidative stress – precursors to cardiovascular disease, DNA damage and possibly cancer.
- Due to the crude recycling process, many pollutants, such as persistent organic pollutants and heavy metals, are released from e-waste, which can easily accumulate in the human body through the inhalation of contaminated air.
- The primitive methods used by unregulated backyard operators (e.g., the informal sector) to reclaim, reprocess, and recycle e-waste materials expose the workers to a number of toxic substances.
New IPCC report warns of dire threat to ocean
Context
The Intergovernmental Panel on Climate Change (IPCC) in its special report underlined the dire changes taking place in oceans, glaciers and ice-deposits on land and sea.
About
- The Intergovernmental Panel on Climate Change (IPCC), the apex referee for scientific evidence on the impact of global warming, in its ‘Special Report on the Ocean and Cryosphere in a Changing Climate’ found that over the 21st century, the ocean is projected to transition to unprecedented conditions with increased temperatures, further ocean acidification, marine heat waves and more frequent extreme El Niño and La Niña events.
- The report was prepared following an IPCC Panel decision in 2016 to determine the impact on the world’s oceans and ice-covered regions.
- Rising seas are already threatening low-lying coastal areas that today are home to 680 million people, about 10 percent of the world’s population.
- The Southern Ocean accounted for 35%–43% of the total heat gain in the upper 2,000 m global ocean between 1970 and 2017, and its share increased to 45%–62% between 2005 and 2017.
- Since the IPCC’s Fifth Assessment Report came out in 2013, scientists have learned a great deal about the impacts of absorbing atmospheric carbon dioxide on the oceans and their denizens, as well as in coastal areas. Unfortunately, the ocean has largely been left out of the discussion on climate.
Evidences of Impacts of Global warming on Oceans
- Since 1993, the rate of warming in the oceans has more than doubled. Melting of the two great ice sheets blanketing Greenland and West Antarctica is speeding up as well, accelerating sea level rise. And West Antarctica’s glaciers may already be so unstable that they are past the point of no return.
- Warming ocean waters are yielding fewer fish and are fueling more intense, rainier tropical storms.
- Ocean heat waves are increasing, threatening corals and other sea life.
- Arctic sea icecontinues to dwindle
Concerns for India
- Global Warming is a concern for all the nations in the world including India.
- A major impact is in the Hindu Kush Himalayan Regions. Floods will become more frequent and severe in the mountainous and downstream areas of the Indus, Ganges and Brahmaputra river basins, because of an increase in extreme precipitation events... the severity of flood events is expected to more than double towards the end of the century.
How to mitigate impacts of global warming on oceans?
- The ocean isn’t just part of the problem; it should be a key part of the solution.
- The 1.5°C report was a key input used in negotiations at Katowice, Poland in 2018 for countries to commit themselves to capping global temperature rise to 1.5°C by the end of the century.
- Researchers find five ways to harness ocean resources to reduce or mitigate global greenhouse gas emissions
- Build offshore wind farms and other ocean-based renewable energy to shift away from dependence on fossil fuels;
- Eliminate carbon emissions from the shipping industry;
- Restore coastal ecosystems such as mangroves and salt marshes, which not only store carbon but also provide myriad benefits, including serving as buffers against tropical storms, filtering pollutants and providing habitat for fish and other wildlife;
- Harvest more ocean-based protein sources, which have a much lower carbon footprint than any land-based animal protein;
- Store carbon in the seafloor, which theoretically has high potential for mitigating greenhouse gas emissions, but also a lot of uncertainty in terms of its environmental impact.
Conclusion
- None of these potential solutions are new concepts, but there has been little political will to invest in the necessary research and development to move them forward.
- These actions may finally get some traction in December, when nations head to Santiago, Chile, for COP25, the annual meeting to review progress of the U.N. Framework Convention on Climate Change.
- At the COP21 meeting in Paris in December 2015, 195 nations signed on to the 2015 Paris climate agreement, pledging to reduce global greenhouse gas emissions enough to keep global warming to “well below” 2 degrees C above preindustrial times. The United States was one of the signing nations, but President Donald Trump has said he plans to withdraw the country from the accord in 2020.
Emissions Gap Report 2019
Context
The 2019 UN Environment Programme (UNEP) Emissions Gap Report paints a “bleak” picture of accelerated global greenhouse gas (GHG) emissions and a growing gap between “what we need to do and what we are actually doing to tackle climate change.”
About
- This is the tenth edition of the United Nations Environment Programme (UNEP) Emissions Gap Report.
- It provides the latest assessment of scientific studies on current and estimated future greenhouse gas (GHG) emissions and compares these with the emission levels permissible for the world to progress on a least-cost pathway to achieve the goals of the Paris Agreement.
- This difference between “where we are likely to be and where we need to be” has become known as the ‘emissions gap’.
What is the “Emissions Gap”?
- The Emissions Gap could also be called the “Commitment Gap”.
- It measures the gap between what we need to do and what we are actually doing to tackle climate change.
- The gap is the difference between the low levels of emissions that the world needs to drop to, compared with the projected level of emissions based on countries’ current commitments to decarbonisation.
Why does the Emissions Gap Matter?
- The gap is important because if we can’t close it and meet the emissions reduction target, we will face increasingly severe climate impacts worldwide.
- It is important that policymakers, and their citizens, know what the gap is so that the commitments countries are making are sufficient to close the gap.
What does the Emissions Gap Report measure?
The Emissions Gap Report measures and projects three key trend lines:
- The amount of greenhouse gas emissions every year up to 2030
- The commitments countries are making to reduce their emissions and the impact these commitments are likely to have on overall emission reduction
- The pace at which emissions must be reduced to reach an emission low that would limit temperature increase to 1.5oC, affordably.
The key “headline” messages and conclusions of the report
- GHG emissions continue to rise, despite scientific warnings and political commitments;
- To close the emissions gap by 2030, annual emissions need to be 15 GtCO2e lower than current unconditional NDCs imply for the 2°C goal, and 32 GtCO2e lower for the 1.5°C goal;
- Enhanced action by G20 members will be essential for the global mitigation effort. Collectively, G20 members – who account for 78% of global GHG emissions – are on track to meet their limited 2020 Cancun Pledges, but seven countries are currently not on track to meet their 2030 NDC commitments.
- “Dramatic strengthening” of the NDCs is needed in 2020. Countries must increase their NDC ambitions threefold to achieve the “well below 2°C” goal and more than fivefold to achieve the 1.5°C goal.
- Although the number of countries announcing net zero GHG emission targets for 2050 is increasing, only a few countries have so far formally submitted long-term low-emission development strategies to the UNFCCC.
- Decarbonising the global economy will require fundamental structural changes, which should be designed to bring multiple co-benefits for humanity and planetary support systems.
- Renewable and energy efficiency, in combination with electrification of end uses, are key to a successful energy transition and to driving down energy-related CO2 emissions.
- Demand-side material efficiency offers substantial GHG mitigation opportunities that are complementary to those obtained through an energy system transformation.


