

11th October 2022 (8 Topics)
Context
The recent WHO allegation saying cough syrups manufactured by an Indian firm could potentially be linked to the death of 66 children in Gambia, raises the burning crucial questions related to drug regulation in the country.
What was the reason for death of Children? (Synopsis of the issue)
- The syrup was found by WHO to be adulterated with diethylene glycol (DEG) and ethylene glycol.
- These chemicals are toxic to humans, and can result in abdominal pain, vomiting, diarrhoea, inability to pass urine, headache, and altered mental state.
- It can also lead to acute kidney injury that can prove fatal in children.
What are the present Drug regulations in India?
Drug regulation in India is a complex process managed by law, mainly the Drugs and Cosmetics Act of 1940, and by multiple ministries, including the Ministry of Health and Family Welfare. The law creates a web of regulatory authorities to govern the process at both the central and the state level.
At Centre level:
- At the central level, the Drugs and Cosmetics Act, 1940, has created the Central Drugs Standard Control Organisation (CDSCO), within which the Drugs Controller General of India (DCGI) is the key regulatory authority, acting under the advice of the Drug Technical Advisory Board (DTAB) and the Drug Consultative Committee (DCC).
- CDSCO operates through zonal offices spread across the country, which have designated roles in drug regulation, such as inspections, recalls, and market surveillance.
- CDSCO also has a role in overseeing the functioning of state authorities involved in drug regulation.
At State Level:
- There exist State Drug Regulatory Authorities (SDRAs), which are statutory bodies created under the Drugs and Cosmetics Act, 1940.
- Falling under the ambit of the respective Health Departments of each state, SDRAs are tasked with limited aspects of drug regulation.
- SDRAs are often conjoined with the food regulation department under the Food and Drug Administration (FDA) in that state, which complicates the proper demarcation of regulatory responsibilities.
What is the Process of drug regulation?
- CDSCO has been entrusted with the responsibility for the approval of new drugs, and the conduct of clinical trials in the country, as well as laying down the standards for drugs.
- It also controls the quality of imported drugs, oversight over the SDRAs and an advisory role in ensuring uniformity in the enforcement of the Drug Control Act.
- Applications for approval of New Drugs are evaluated by the 12 Subject Expert Committee (SEC) (formerly referred to as New Drug Advisory Committees (NDAC)), consisting of experts usually drawn from Government Medical Colleges and Institutes across India.
|
The Drugs and Cosmetics Act also has provisions to compensate the families of victims of adulteration. It calls for the penalty to be extracted from a convicted manufacturer and given to families of the victims. |
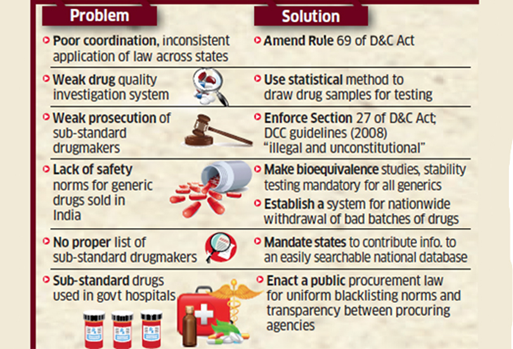
What are the challenges associated with Indian drug regulations?
- Non-scientific classification of offence: The distinction between minor and major offenses isn’t scientific in India. States do not prosecute dissolution, disintegration, or impurity failures because they deem them minor offense.
- Liberal punishments: lack of adequate fines and punishment has made the crime of Pharma sectors more prominent.
- Competition between states to boost the Pharma sector: As government of India has made pharma sector under innovation criteria for rankings among states has led it under a competition to establish drugs and pharma industry.
- Interstate variation in the prosecution: The variations among the States’ regulation against drug control and Quality check makes it difficult to analyse the real problem.


