

Context
A new study shows a way to use calcium-41 the same way carbon-14 has been used in carbon-dating, but with several advantages.
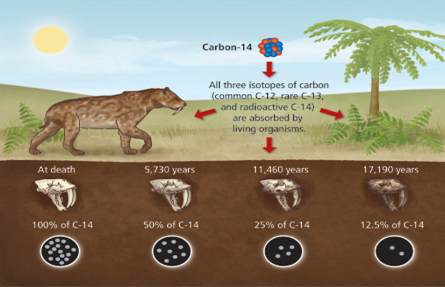
What is Radiometric dating?
- Radiometric dating is a method used to determine the age of organic materials by measuring the decay of radioactive isotopes present in them.
- Specifically, carbon-14 dating is a commonly used radiometric dating technique for estimating the age of once-living organisms.
- When an organism is alive, it absorbs and loses carbon-14 atoms through various biological processes.
- However, once the organism dies, the intake of carbon-14 ceases, and the existing carbon-14 begins to decay.
- By comparing the relative abundance of carbon-14 in the remains of the organism with the expected amount, scientists can estimate the time of death.
What is the issue with this method?
|
Carbon-14 has a half-life of 5,700 years, so the carbon dating technique can’t determine the age of objects older than around 50,000 years. |
- A significant early issue with the method was to detect carbon-14 atoms, which occur once in around 1012carbon atoms.
- Calcium-41 is rarer, occurring once in around 1015calcium atoms.
- In the new study, researchers pitched a technique called atom-trap trace analysis (ATTA) as a solution.
- ATTA is sensitive enough to spot these atoms; specific enough to not confuse them for other similar atoms; and fits on a tabletop.
- In ATTA, a laser’s frequency is tuned such that it imparts the same energy as required for an electron transition in calcium-41.
- The electrons absorb and release this energy, revealing the presence of their atoms.


