

Context
Recently, the Nobel Prize in chemistry, 2022 was awarded to scientists Carolyn R. Bertozzi, Morten Meldal and K. Barry Sharpless for their development of ‘click chemistry’ and ‘bioorthogonal chemistry’.
So let us assess their applications of ‘Click chemistry’ and understand how it works.
About
- Click chemistry is a method for attaching a ‘probe’ or ‘substrate’ of interest to a specific biomolecule, a process called bio-conjugation.
- The possibility of attaching fluorophores and other reporter molecules has made click chemistry a very powerful tool for identifying, locating, and characterizing both old and new biomolecules.
|
Fluorophores are microscopic molecules, which may be proteins, small organic compounds, or synthetic polymers that absorb light of specific wavelengths and emit light of longer wavelengths. |
- One of the earliest and most important methods in bioconjugation was to express a reporter on the same open reading frame as a biomolecule of interest.
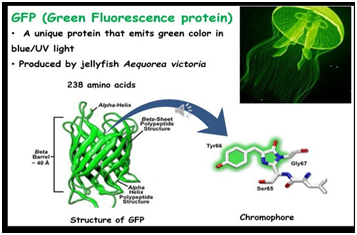
- Notably, GFP was first (and still is) expressed in this way at the N- or C- terminus of many proteins. However, this approach comes with several difficulties.
- For instance, GFP is a very large unit and can often affect the folding of the protein of interest.
- To overcome these challenges, chemists have opted to proceed by identifying pairs of bioorthogonal reaction partners, thus allowing the use of small exogenous molecules as biomolecular probes.
|
Bio-orthogonal Chemistry:
|
Analysis
What are its applications?
- Click chemistry, is a way of building molecules like snapping Lego blocks together.
- It takes two molecules to click, so researchers refer to each one as ‘click partners’.
- It is a term that was introduced by B. Sharpless in 2001 to describe reactions that are high yielding, wide in scope, create only by-products that can be removed without chromatography, are stereospecific, simple to perform, and can be conducted in easily removable or benign solvents.
- The click reaction has proven to be very useful for modifying functional biomolecules because of its high chemoselectivity.
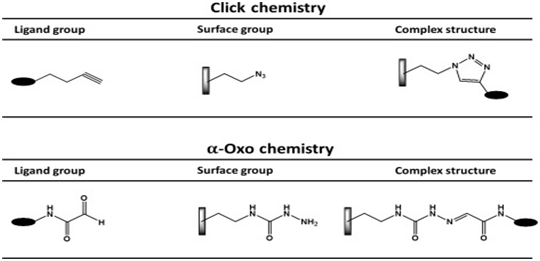
- Biologic oligomers and polymers, such as peptides, nucleic acids, and carbohydrates, have been modified by using the copper-catalyzedazide-alkyne cycloaddition click reaction.
How it works?
- Click chemistry is the 1,3-dipolar cycloaddition of an azide and alkyne to form 1,2,3-triazole, which has been applied for a wide range of applications due to its simple workup and purification steps, rapidly creating new products.
- For a reaction to be considered a click reaction, it must satisfy certain characteristics:
- modularity
- insensitivity to solvent parameters
- high chemical yields
- insensitivity towards oxygen and water
- regiospecificity and stereo specificity
- A large thermodynamic driving force (>20 kcal/mol) to favour a reaction with a single reaction product. A distinct exothermic reaction makes a reactant "spring-loaded".
- The process would preferably:
- have simple reaction conditions
- use readily available starting materials and reagents
- use no solvent or use a solvent that is benign or easily removed (preferably water)
- provide simple product isolation by non-chromatographic methods (crystallisation or distillation)
- Have high atom economy.
Is Click Chemistry irreversible?
- ‘Click chemistry’ allows for the linking together of chemical modules, however, there are currently no methods that also allow for facile 'de-clicking' to unlink them.
Additional applications include:
- Two-dimensional gel electrophoresis separation
- Modification of natural products and pharmaceuticals
- Drug discovery
- Modification of DNA and nucleotides by triazole ligation
- Polymers and biopolymers
- Surfaces
- Material science
- Nanotechnology etc.
Limitations:
Limitations emerge from the chemistry of the probe to its target. In order for this technique to be useful in biological systems, click chemistry must run at or near biological conditions, produce little and (ideally) non-toxic byproducts, have (preferably) single and stable products at the same conditions, and proceed quickly to high yield in one pot.


