A new analysis of California's Monterey Bay evaluates kelp's potential to reduce ocean acidification, the harmful fallout
Context
A new analysis of California's Monterey Bay evaluates kelp's potential to reduce ocean acidification, the harmful fallout from climate change on marine ecosystems and the food they produce for human populations.
Background
- A new on-site, interdisciplinary analysis of giant kelp in Monterey Bay off the coast of California sought to further investigate kelp's acidification mitigation potential.
- The findings show that near the ocean's surface, the water's pH was slightly higher, or less acidic, suggesting the kelp canopy does reduce acidity.
- However, those effects did not extend to the ocean floor, where sensitive cold-water corals, urchins and shellfish dwell and the most acidification has occurred.
|
Monterey Bay, California
|
Analysis
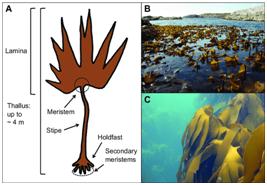
What is Kelp?
- Kelp is the largest and fastest-growing marine algae or seaweed.
- It belongs to the brown algae group known as
- Although kelp may resemble an underwater plant, it is in fact a protist, the same family of organisms as moulds and amoebas.
- Kelp forests are found in the temperate and polar coastal regions of the world.
- Four species of kelp are found around the South African coast, with Ecklonia maximabeing the most familiar, often being washed up on beaches following heavy storms.
- Three of these species, namely sea bamboo (Ecklonia maxima), split-fan kelp (Laminaria pallida) and bladder kelp (Macrocystis pyrifera) can be seen in the Kelp Forest Exhibitat the Two Oceans Aquarium.
|
Important facts |
|
|
Scientific name |
Laminariales |
|
Class |
Phaeophyceae |
|
Phylum |
Ochrophyta |
|
Higher classification |
Brown algae |
|
Order |
Laminariales |
|
Domain |
Eukaryota |
How is it similar to plant?
- Like plants, kelp needs sunlight to photosynthesise and convert carbon dioxide into sugars.
- Unlike plants, however, kelp does not use roots to extract nutrients from the soil - kelp can extract the needed nutrients directly from the water around it.
- So, instead of a "root system" it has a modified anchoring system known as a holdfast.
What is ocean acidification?
- Ocean acidification refers to the process of our planet's oceans becoming more acidic due to the global increase in carbon dioxide emissions.
- Since the Industrial Revolution, experts estimate that Earth's oceans have absorbed more than a quarter of the atmospheric carbon dioxide(CO2) released from the burning of fossil fuels.
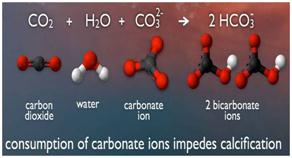
- Once in the ocean, the dissolved carbon dioxide undergoes a series of chemical reactions that increase the concentration of hydrogenions while lowering the ocean's pH and carbonate minerals — a process called ocean acidification.
What causes ocean acidification?
- When carbon dioxide from the atmosphere is dissolved in seawater, it forms carbonic acid and releases hydrogen ions.
- These hydrogen ions bond with available carbonate ions (CO3-) to form bicarbonate (HCO3-), depleting the available carbonate in the oceans.
- Calcium carbonate minerals are the building blocks for the skeletons and shells of many marine organisms. In areas where most life now congregates in the ocean, the seawater is supersaturated with respect to calcium carbonate minerals.
- Calcium carbonate minerals are the building blocks for the skeletons and shells of many marine organisms. In areas where most life now congregates in the ocean, the seawater is supersaturated with respect to calcium carbonate minerals.
- Less carbonate in the oceans makes it more difficult for calcifying creatures such as corals, clams, sea urchins or plankton to form their calcium carbonate (CaCO3) shells or skeletons.
|
Impact of ocean acidification
- Altering ecosystem: The long-term absorption results in a shift in ocean carbonate chemistry, which has the potential to alter biogeochemical cycles and ecosystem function in the future.
- Altering availability of food resources: Sundarbans, one of the most biologically rich ecosystems also serves as the spawning ground of rich coastal fisheries of Bay of Bengal. Ocean acidification can severely alter the availability of food resources (e.g. plankton) required for coastal fish populations to thrive. This can ultimately lead to a crash of coastal fisheries and livelihood in the region.
- Impact on nitrogen cycle: Ocean acidification has the potential to alter the marine nitrogen cycle which controls much of primary production in the sea.
- Increased vulnerability of coastal ecosystem: Moreover, many fragile coastal ecosystems such as coral reefs, mangroves and lagoons will become vulnerable and reel from the effects of ocean acidification due to change in coastal water carbonate chemistry and thus leading to loss of biodiversity.
- Deteriorating ocean’s health: It could also lead to catastrophic consequences for ocean health and also for tourism of Bay of Bengal nations including India.
Why does kelp matter?
- Kelp is a keystone organism, which means its role in the ecosystem is so vital, that without it the ecosystem would collapse.
- Kelp forests are among the most productive ecosystems in the world
- Due to climate change and elevated sea temperatures, the environment for kelp to successfully grow in is at risk. This poses a huge threat to biodiversity within the ocean
- Kelp purifies water and removes waste products produced by the animals living within the forests
- Underwater forests provide shelter, food and the ideal habitat for various species
- Commercially, kelp is used in a wide variety of products, from salad dressings, cosmetics, food, vitamin supplements, skin care products, paint, etc.
Conclusion
The fast changes are stressing out the entire marine ecosystem. The future holds even more challenges. By 2050, scientists predict that 86 percent of the world’s ocean will be warmer and more acidic than anything in modern history. By 2100, the pH of the surface ocean could drop to under 7.8, or more than 150 percent compared to today’s already-corrosive state—and potentially even more, in some particularly sensitive parts of the planet.