

Context
A recent Parliamentary panel Report stated that the Central Drugs Standard Control Organization (CDSCO) is falling short in effectively regulating the medical devices industry.
About
- The parliamentary panel has presented its 138th report on “Medical Devices: Regulations and Control” pertaining to the department of health and family welfare to the head of Rajya Sabha and Lok Sabha.
- The committee has observed that the function of the Central Drugs Standard Control Organization (CDSCO) primarily focus on the regulation of drugs as a regulatory body was set up originally to regulate pharma and other Medical Devices segment as well.
- The Medical devices Rule (MDR) 2017 mandated the CDSCO to regulate medical devices as well. But the organization in its existing structure and expertise (which is more pharma centric) is unable to regulate the medical devices industry.
|
Central Drugs Standard Control Organization (CDSCO):
Major functions of CDSCO:
|
Committee Observation:
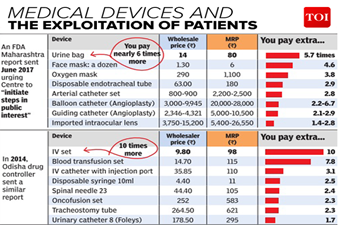
- Insufficient Medical Device Testing Laboratories: The country has only 18 certified Medical Device Testing Laboratories that have been approved by CDSCO and it is minuscule keeping in view the size of the country.
- Underuse of Labs: The institutes which have high-tech labs are not being used and are not allowed to be used to test medical devices for their electronic, electromagnetic, and biochemical-run aspects.
- Absence of Research Ecosystem: Indian Medical Devices Industry presently lacks a research ecosystem and infrastructure for manufacturing of high tech, advanced medical devices (Class C&D)
- Adequate common infrastructure: Accredited laboratories in various regions of the country for standard testing can encourage local manufacturers to get their products tested for standards. It may reduce the cost of production which ultimately will improve the availability and affordability of medical devices in the domestic market.
- Low Standards: The Indian Medical Devices Industry doesn’t have the facilities to produce such medical devices comparable to global standards.
Recommendations of Committee
- Post-market surveillance system for Medical Devices: The Committee noted that there is a dire need for developing IT enabled feedback-driven post-market surveillance system for Medical Devices to evaluate the efficiency of specific Medical Devices.
- Need for Medical device registry: This is to ensure the traceability of patient who has received the implant, to assess the performance of the implant also to seek feedback on the functional capacity of medical devices.
- Imparting necessary skill-set: The union government and the State governments must work in coherence and impart the necessary skills to the local medical device officers and also devise a mechanism to regularly designate State Medical personnel so that the mandate of the legislation can be implemented effectively.
- Ensuring Participation of IITs/IISC etc: The Ministry should allow the new regulator to involve institutions such as IISC, CSIR, DRDO, and the network of IITs to test medical devices for safety and efficacy. These institutes have high-tech labs and thus can be used to test medical devices for their electronic, electromagnetic, and biochemical-run aspects.
- Single Window System: The multiplicity of regulations is creating chaos. So, a single window clearance would significantly boost investment in R&D in the field of medical devices and would also reduce the time required for obtaining approvals from different Departments/Ministries.
- Research Linked Incentive (RLI) Scheme: Indian Medical Devices Industry lacks the infrastructure for manufacturing advanced medical devices. It is recommended to start a Research Linked Incentive (RLI) Scheme in Line with the PLI scheme.
- Improving on Research and Development: Indian Medical Devices Industry also lacks the research ecosystem for manufacturing high medical devices (Class C&D). We need to inculcate a culture of research and development in medical devices in institutions of excellence.
Significance:
- Encourages Local Manufacturers: Having adequate common infrastructure including accredited laboratories in various regions of the country for standard testing would significantly encourage local manufacturers to get their products tested for standards.
- Lower Cost of Production: The measures undertaken would also help in reducing the cost of production which ultimately will improve the availability and affordability of medical devices in the domestic market.
- Matching with the pace of industry growth: The industry is growing by leaps & bounds, and if the medical device regulations are dispensed with by qualified and well-trained Medical Device Officers to give a fillip to the Medical Device industry in the country.


