Context
Recently, the U.S. Food and Drug Administration approved two treatments namely, Casgevy and Lyfgenia, for the treatment of sickle cell disease (SCD) in patients.
About
About the information:
- These are the First Gene therapies approved for the treatment of sickle cell.
- The cell-based gene therapies were approved for the treatment of sickle cell disease (SCD) in patients 12 years and older.
- Additionally, one of these therapies, Casgevy, is the first FDA-approved treatment to utilize a type of novel genome editing technology, signaling an innovative advancement in the field of gene therapy.
How these treatments work?
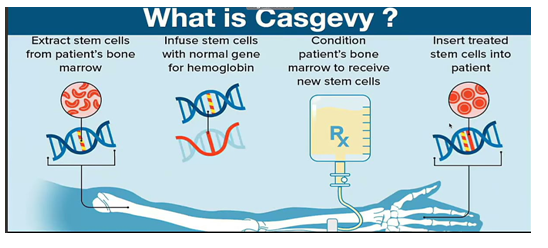
- Casgevy, a cell-based gene therapy, is approved for the treatment of sickle cell disease in patients 12 years of age and older with recurrent vaso-occlusive crises.
- Casgevy is the first FDA-approved therapy utilizing CRISPR/Cas9, a type of genome editing technology.
- Patients’ hematopoietic (blood) stem cells are modified by genome editing using CRISPR/Cas9 technology.
- CRISPR/Cas9 can be directed to cut DNA in targeted areas, enabling the ability to accurately edit (remove, add, or replace) DNA where it was cut.
-
- The modified blood stem cells are transplanted back into the patient where they engraft (attach and multiply) within the bone marrow and increase the production of fetal hemoglobin (HbF), a type of hemoglobin that facilitates oxygen delivery. In patients with sickle cell disease, increased levels of HbF prevent the sickling of red blood cells.
- Lyfgenia is a cell-based gene therapy:
- Lyfgenia uses a lentiviral vector (gene delivery vehicle) for genetic modification and is approved for the treatment of patients 12 years of age and older with sickle cell disease and a history of vaso-occlusive events.
- With Lyfgenia, the patient’s blood stem cells are genetically modified to produce HbAT87Q, a gene-therapy derived hemoglobin that functions similarly to hemoglobin A, which is the normal adult hemoglobin produced in persons not affected by sickle cell disease.
- Red blood cells containing HbAT87Q have a lower risk of sickling and occluding blood flow. These modified stem cells are then delivered to the patient.
Sickle Cell Disease:
- Sickle cell disease is a group of inherited blood disorders affecting approximately 100,000 people in the U.S.
- It is most common in African Americans and, while less prevalent, also affects Hispanic Americans.
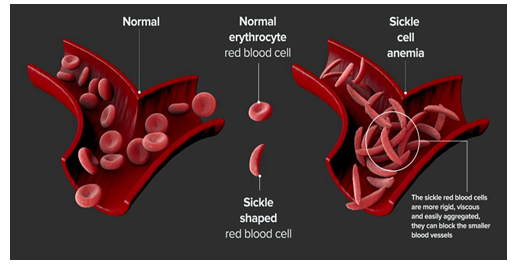
- The primary problem in sickle cell disease is a mutation in hemoglobin, a protein found in red blood cells that deliver oxygen to the body’s tissues.
- This mutation causes red blood cells to develop a crescent or “sickle” shape.
- These sickled red blood cells restrict the flow in blood vessels and limit oxygen delivery to the body’s tissues, leading to severe pain and organ damage called vaso-occlusive events (VOEs) or vaso-occlusive crises (VOCs).
- The recurrence of these events or crises can lead to life-threatening disabilities and/or early death.
|
The US Food and Drug Administration (FDA)
|