

Context:
A recent report by a public health group on pharmaceutical marketing practices has revealed widespread use of bribes and inducement by Pharma companies to the doctors in order to increase the sale of their products. For doctors the Medical Council of India has a code of ethics which bars them from accepting any gifts, cash, travel facilities or hospitality from Pharma companies. However for the pharmaceutical companies there is a voluntary code known as Uniform Code of Pharmaceutical marketing practices or UCPMP which experts says is a not a very effective mechanism to check the prevailing malpractices. So what is the best way to tackle this issue?
Background
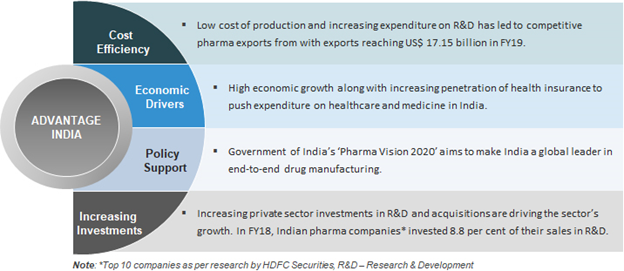
- The pharmaceutical sector was valued at US$ 33 billion in 2017.
- The country’s pharmaceutical industry is expected to expand at a CAGR of 22.4 per cent over 2015–20 to reach US$ 55 billion. India’s pharmaceutical exports stood at US$ 17.27 billion in FY18 and have reached US$ 19.14 billion in FY19.
- India is the largest provider of generic drugs globally. Indian pharmaceutical sector industry supplies over 50 per cent of global demand for various vaccines,
Edited excerpts from the debate
Question: What are the various Pharmaceutical Marketing Malpractices?
The pharmaceutical industry has been accused of adopting questionable practices in relation to the marketing of their products. The main focus of attention in this respect has been the suspect interactions between pharmaceutical companies and healthcare practitioners/ providers (HCPs). The unethical marketing practices comprises of:
Claims made during promotional activities that are:
- Misleading and give rise to unjustifiable drug use leading to risks.
- Not capable of substantiation.
- Not in good taste.
- Comparative with another drug, without any substantive basis for such comparison.
- Unqualified in the use of terms such as safe.
- An improper representation of the true nature of the drug.
Interaction with HCPs comprises of following malpractices
- Free samples of drugs
- Offering of gifts or monetary benefits to HCPs or family members.
- Providing travel or lodging facilities to HCPs in relation to attending seminars, continuing medical education (CME) programmes.
- Extension of grants or funds for medical research or clinical trials.
Question: What are the various laws and rules in place for doctors and Pharmaceutical companies for preventing these marketing malpractices?
- Code of ethics for doctors recommended by MCI to prevent accepting any bribes or inducements from pharma companies for prescribing specified pharmaceuticals.
- Draft Pharmaceutical Policy, 2017 also notes that unethical practices deployed by pharma companies are an area of concern.
- There is no law at present that regulates the promotion and marketing of drugs (including medical devices) by companies in front of HCPs.
- Interactions between pharma companies and HCPs are at best limited under restrictions cast on HCPs under the Medical Council of India (“MCI”) regulations. The Drugs and Cosmetics Act, 1940 and the Drugs and Cosmetics Rules, 1945 regulates what pharmaceutical companies can and cannot print on their product labels, but the legislation falls short in terms of actual regulation of interactions with HCPs – what companies can and cannot say, or give, to HCPs. On the other hand, Direct to Consumer advertising is controlled under the Drugs and Magic Remedies (Objectionable Advertisement) Act of 1954.
- Faced with increasing complaints on improper marketing practices employed by pharma companies and lobbying by patient advocacy groups, the Government introduced the Uniform Code of Pharmaceutical Marketing Practices (UCPMP) – this was but a guidance document for the industry, voluntary at first and lacking the necessary regulatory teeth to have force of law. That said, the industry has adopted the same and we have seen a lot of advisory going out in this regard.
- The UCPMP sets out a level of regulation in that it ensures that:
- Claims for usefulness, novelty and safety are based on up-to-date scientific data and credible evidence.
- Comparison of drugs should be fair and free from comparative disparagement.
- Promotional materials to have minimum prescribed levels of information so as to enable an HCP to exercise his/ her discretion based on the same. Materials to be an honest and accurate representation of the qualities of the drug. Transparency of disclosure to be made in respect of paid journal publications.
- Control on free samples. Better accountability. Samples are not gifts or freebies.
- Restriction on quid–pro-quo arrangements. No gifts for personal benefits.
- No free travel or vacations.
- No individual monetary grants or funding save and except through modalities laid down by law in a transparent manner. Full disclosure.
- The UCPMP is India’s version of the US PhRMA Code and the Physicians Payment of Sunshine Act. Interestingly enough, both these legislations have been enforced by the authorities and have resulted in hundreds of millions of dollars in fines for some pharma companies that were found to be in violation of these regulations. In India, the UCPMP is voluntary.
- The UCPMP suffered from a serious lack of the proverbial teeth. To effectuate more rigorous regulatory control over marketing activities of the industry, therefore, the Government has been contemplating introduction of the Draft Essential Commodities (Control of Unethical Practices in Marketing of Drugs) Order, 2017 (CUPMD Order). While it surfaced online sometime earlier this year, surprisingly the same has now been removed from the public domain by the Government.
Question: Why Pharmaceutical companies are resorting on medical representatives not for technical education but for extending inducement to doctors?
Marketing is the main task of any business and it does not involve bribing doctors. It involves a large team responsible for communication and marketing of medical products. Medical representatives are educating the medical practitioners on new products of the pharma companies and it is being done under rules and ethics.
Question: Why Pharmaceutical companies do not strictly follow the voluntary code?
- According to the Pharmaceutical Association the Uniform Code of Pharmaceutical Marketing Practices (UCPMP) is voluntary. It is taking all the steps to set the industry in order. Whenever it is receiving the complaints directly by doctors or citizens or from government we investigate it and redress the grievances.
- However, the UCPMP suffered from a serious lack of the proverbial teeth. To effectuate more rigorous regulatory control over marketing activities of the industry, therefore, the Government has been contemplating introduction of the Draft Essential Commodities (Control of Unethical Practices in Marketing of Drugs) Order, 2017 (CUPMD Order). While it surfaced online sometime earlier this year, surprisingly the same has now been removed from the public domain by the Government due to the industry lobbying.
Question: Why Pharmaceutical companies spending on marketing is 200 or 300 percent higher than the spending on R&D?
According to the Pharmaceutical Industry Association the marketing is a part of the pharmaceutical business like any other business. It is a part of a manufactured product. To make a sale it must be there and it is an indispensable component. However, on the other it does not mean that marketing costs include bribes or inducements to doctors. Research and Development is an exercise that depends on the margins of the products sale. After rationalization of margins we as an industry are unable to spend more on R&D. Still we have expanded our exports and manufacturing units around the world due to our better service and R&D.
Question: Is self-regulation by Pharmaceutical companies enough even after marketing malpractices were reported by credible reports? Is government thinking to regulate it?
- No doubt self-regulation of the marketing of pharmaceuticals has not worked properly and various reports have reported several malpractices in the process. To effectuate more rigorous regulatory control over marketing activities of the industry, therefore, the Government has been contemplating introduction of the Draft Essential Commodities (Control of Unethical Practices in Marketing of Drugs) Order, 2017 (CUPMD Order). While it surfaced online sometime earlier this year, surprisingly the same has now been removed from the public domain by the Government due to the industry lobbying.
- Ethics is another way where both industry and health practitioners must come together to minimize malpractices.
Question: Does prescription of generic medicines is going to eliminate pharmaceutical marketing malpractices?
Recently, Prime Minister, Narendra Modi has created a momentum across the country by motivating the industry and health practitioners towards generic medicines production and prescription respectively. This will highly minimize the Pharmaceutical Marketing Malpractices in the country.
More Articles

