16th October 2024 (13 Topics)
Context
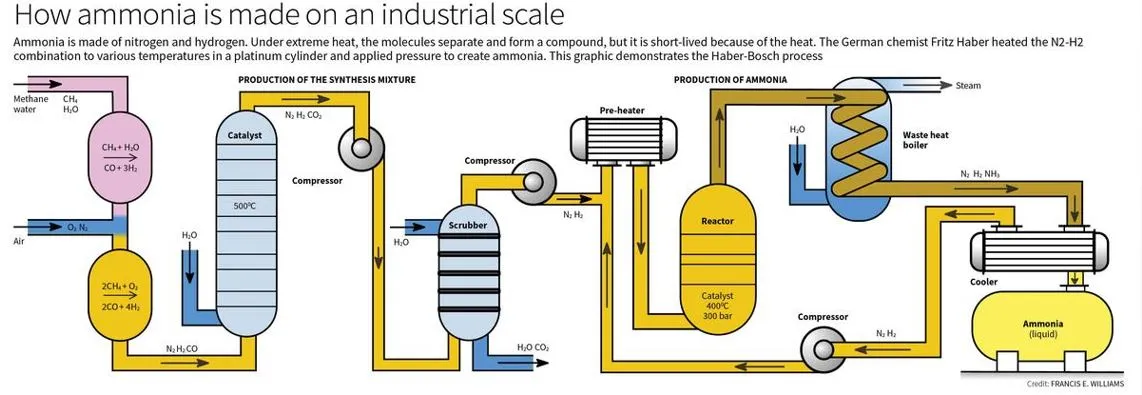
Recent reports emphasize the significance of the Haber-Bosch process in converting atmospheric nitrogen into fertilizer, addressing the growing global food demand while highlighting the disparity between industrial and natural nitrogen replenishment methods.
What Is Nitrogen?
- Nitrogen is a key element that makes up about 78% of the Earth's atmosphere, primarily in the form of molecular nitrogen (N2). Although abundant, this form of nitrogen is not usable by plants because it exists as a stable triple bond between two nitrogen atoms, making it inert and difficult to break apart.
- The Nitrogen Cycle: Nitrogen enters the soil in forms that plants can use, known as reactive nitrogen, through several natural processes:
- Lightning: When lightning strikes, it provides enough energy to break the nitrogen triple bond, creating nitrogen oxides (NO and NO2). These oxides can mix with water to form nitric acid, which falls as rain and enriches the soil.
- Bacteria: Certain bacteria, like Azotobacter and Rhizobia, can convert atmospheric nitrogen into reactive forms. Rhizobia live in the roots of legume plants, helping them absorb nitrogen while receiving nutrients in return.
- Aquatic Plants: Some aquatic ferns, like Azolla, work with bacteria to convert nitrogen into a usable form, acting as a natural fertilizer when they decay.
The Role of Ammonia
- Ammonia (NH3) is a key reactive form of nitrogen that plants need for growth. It can be produced through natural processes or industrial methods.
- The Haber-Bosch process is a significant industrial method that synthesizes ammonia from nitrogen and hydrogen gases under high pressure and temperature.
The Haber-Bosch Process
- Development: It is developed by Fritz Haber and later improved by Carl Bosch, the Haber-Bosch process revolutionized ammonia production. It requires:
- High temperatures (around 200°C)
- High pressures (up to 200 atm)
- A catalyst (initially osmium, later iron oxides)
- Impact: This process enables the mass production of synthetic fertilizers, which have dramatically increased global food production, allowing us to meet the demands of a growing population.
Environmental Concerns
While the Haber-Bosch process has been crucial for food security, it also has significant downsides:
- Excess Nitrogen: The widespread use of nitrogen fertilizers leads to soil saturation, where plants absorb more nitrogen than they need. This excess can:
- Acidify rain, harming ecosystems.
- Cause runoff into water bodies, leading to algal blooms that deplete oxygen and harm aquatic life.
- Food Security Issues: Despite increased food production, issues like hunger and malnutrition persist due to economic and political factors, illustrating that technology alone cannot solve these problems.
More Articles