

India is facing shortages of the two monoclonal antibody therapies the Itolizumab and Tocilizumab.
Context
India is facing shortages of the two monoclonal antibody therapies the Itolizumab and Tocilizumab.
About
About the Monoclonal antibodies
- The immune system is able to create antibodies which are tiny Y-shaped proteins in our blood.
- They recognise microbial enemies and bind to them and signals the immune system to launch an attack on the pathogen.
- They recognise microbial enemies and bind to them and signals the immune system to launch an attack on the pathogen.
- Monoclonal antibodies are artificially created antibodies which aim to aid the body’s natural immune system.
- They target a specific antigen which is a protein from the pathogen that induces immune response.
What is monoclonal antibodies therapy?
- In 1900s, the Nobel-prize winning German immunologist Paul Ehrlich proposed the idea of a ‘Zauberkugel‘ (magic bullet). It is a compound which selectively targets the pathogen.
- Muromonab-CD3 was the world’s first monoclonal antibody to be approved for clinical use in humans.
- Muromonab-CD3 is an immunosuppressant drug that is given to reduce acute rejection in patients with organ transplants.
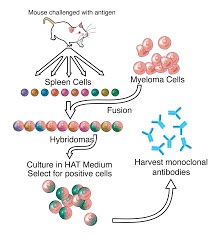
- Monoclonal antibodies are created in the lab by exposing white blood cells to a particular antigen.
- In the case of Covid-19, monoclonal antibodies are produced by using the spike protein of the SARS-CoV-2 virus that facilitates the entry of the virus into the host cell.
- To increase the quantity of antibodies produced, a single white blood cell is cloned, which in turn is used to create identical copies of the antibodies.
About these two therapies
Itolizumab therapy
- It was developed by Bengaluru-based biopharma company Biocon.
- The Drugs Controller General of India had authorised it for “restricted emergency use” in the country in June 2020.
- Itolizumab targets CD6, a protein found in the outer membrane of a T-cell.
- T-cell is a type of white blood cell that plays a central role in the body’s immune response.
- Itolizumab, by binding to CD6, tones down T-cell activation and causes reduction in synthesis of pro-inflammatory cytokines.
Tocilizumab
- This monoclonal antibody has got approved from the FDA to treat rheumatologic disorders.
- This antibody also inhibits IL-6 activity, and was proposed as a potential therapy for severe Covid.
- The drug is currently approved for off-label use in India, which means it can be used after informed consent of patients.
|
Other monoclonal antibody therapies developed worldwide Bamlanivimab and etesevimab
Casirivimab and imdevimab
|


