27th August 2022 (11 Topics)
Context
The concentration of ozone-depleting substances in the atmosphere has reduced to reach a significant milestone this year.
About
- Levels of ozone-depleting substances (ODSs) in 2022 are back to those observed in 1980 before ozone depletion was significant.
- However, the pace of reduction in ODSs over Antarctica, which experiences a large ozone hole in spring, has been slower.
- National Oceanic and Atmospheric Administration (NOAA) Ozone Depleting Gas Index for the Antarctic has fallen 26 per cent from peak values in the 1990s, with recovery of the Antarctic ozone layer projected to occur sometime around 2070.
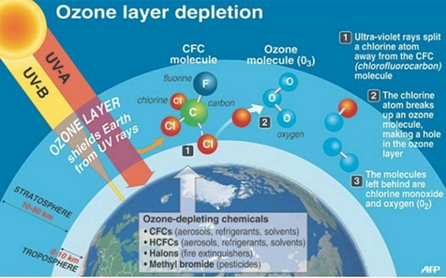
What is Ozone hole?
- An ozone hole is the thinning of the ozone layer boosted in size by colder temperatures.
- As the temperature high up in the stratosphere starts to rise, ozone depletion slows, the polar vortex weakens and breaks down.
- By the end of December, ozone levels return to normal. This time around, however, the process took longer.
- The formation of ozone hole in the Antarctic has been an annual occurrence and has been recorded for the last 40 years.
- Human-made chemicals migrate into the stratosphere and accumulate inside the polar vortex.
- It begins to shrink in size as warmer temperatures dominate
|
Ozone:
|
|
The science behind ozone depletion
|