16th November 2024 (9 Topics)
Context
In a landmark step towards sustainable transportation, India is set to unveil its first hydrogen-powered train in December 2024 (first trial will take place on the Jind-Sonipat route in Haryana), showcasing a revolutionary shift in rail travel.
About Hydrogen-Powered Train
- This zero-emission train, which will operate without diesel or electricity, is part of Indian Railways' broader strategy to reduce its carbon footprint and achieve "net zero carbon emissions" by 2030.
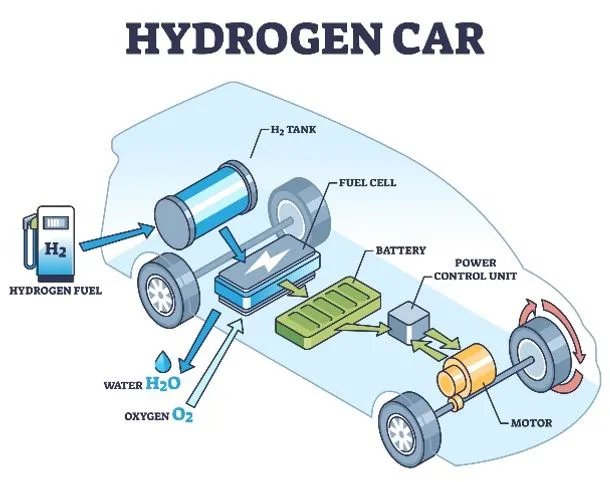
- The hydrogen-powered train operates using hydrogen fuel cells to generate the electricity required to drive its engines.
- Unlike traditional trains powered by diesel or electricity, this train produces only water and steam as byproducts, resulting in zero harmful emissions. Hydrogen and oxygen undergo a chemical reaction in the fuel cells, producing electricity without generating pollutants.
- Compared to diesel trains, hydrogen-powered trains are also much quieter, producing 60% less noise, enhancing passenger comfort.
- Key Features and Trials
- Speed and Distance: The hydrogen train will reach speeds of up to 140 km/h and can travel up to 1,000 kilometers on a single tank of hydrogen fuel, making it suitable for longer distances.
- Water Usage: The train will require approximately 40,000 liters of water per hour to sustain the chemical reaction in the fuel cells.
- Cost and Infrastructure: The cost of each hydrogen-powered train is approximately Rs 80 crore. Indian Railways is investing in the infrastructure required for these trains, including hydrogen storage and refueling stations, essential for supporting widespread use.
Fact Box: Hydrogen Fuel Cell
|